Recently been writing up, every day or two, small groups of companies I ran across in my overview of the respiratory healthtech market [the full list is here] and posting them on LinkedIn.
Thought I’d share this short note about two companies I found working on IPF, which warrants much more attention than it gets. Let me know what you think of the summary, and thanks for reading.
Most people have never heard of idiopathic pulmonary fibrosis (IPF), though it takes the lives of 40k people each year in the US. Roughly the same number as breast cancer, except no one talks about it.
As the name suggests, its causes are a mystery. Frustrating and disheartening to treat, it is, in the words of one physician, “a relentless disease that turns lungs to stone.”
Unfortunately, funding for research on IPF is poor. Drugs that can slow its progression have only recently arrived. Still, more than half die within four years of being diagnosed, a survival rate worse than most cancers.
Thankfully, my recent survey of the respiratory healthtech market turned up at least two companies working on IPF/PF.
The first, IMVARIA, led by Joshua Reicher, M.D. and team, analyzes lung CT scans with AI to help physicians identify and diagnose IPF much earlier on.
That speed is important. On average, it currently takes doctors 2-3 years to diagnose IPF after someone develop symptoms. IMVARIA expects it can reduce this delay, enabling people to start lung-function preserving treatments at an earlier stage.
In January, Fibresolve, the company’s lead AI biomarker, received FDA marketing authorization and Breakthrough Device Designation. Shockingly, this is apparently the first-ever clearance of a diagnostic tool of any type in lung fibrosis.
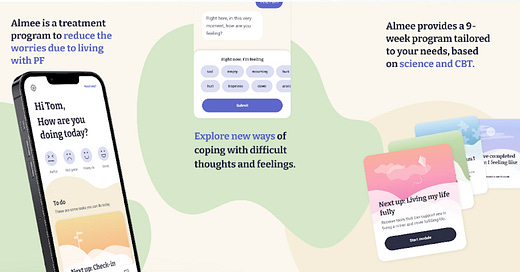
The second product is Almee, which offers a 9-week program of digital cognitive behavioral therapy (CBT) specifically focused on the treatment of anxiety symptoms related to pulmonary fibrosis.
It’s the result of a collaboration between Alex Therapeutics and Vicore Pharma AB designed to target the significant psychological impact of the disease. No surprise that an unforeseen and deadly diagnosis, which progressively limits physical functioning, can also have a terrible mental burden.
Almee was also granted FDA Breakthrough Device Designation, after a recent clinical trial showed its effectiveness in reducing anxiety symptoms and improving health-related quality of life.
With more than 250 thousand Americans living with IPF/PF, and its incidence on the rise, I’m grateful that companies are exploring new options to help people get more timely care and life-improving treatment 🙏